Calculate E Cell for the Following Reaction
E cell 198V. Predict the cell potential for the following reaction when the pressure of the oxygen gas is 250 atm the hydrogen ion concentration is 010 M and the bromide ion concentration is 025 M.

Cell Potential Problems Electrochemistry Youtube
2Cl aq calculate the standard reduction potential for the following the half reaction at 25C.

. The viscosity of methane at 20 C and 1 atm pressure is 109x10-4 Calculate the average collision. E cell E o cell - 00257n ln Q or in terms of log 10. Write the cell reaction and calculate E.
Write the cell reaction and calculate Eo cell of the following electrochemical cell. 2 Ag aq Ni s 2 Ag s Ni2 2 aq E cell 094 V The forward reaction is spontaneous. Given E 136 V for the reaction Cl 2 g 2e.
2Fe2 aq Cd2 aq 2Fe3 aq Cd s 6 A 117 V B-117 V C-037Vv D 037 V E None of these. Calculating Standard Cell Potentials. Chemical reaction is a process in which a chemical compound converts to different chemical compound question_answer Q.
Up to 256 cash back Calculate to determine whether the following redox reactions are spontaneous as Written. Calculate E cell for the following reaction. Show transcribed image text Determine the cell reaction the standard cell potential and the half-cell that acts as the cathode for the voltaic cells composed of the following half-cells.
Calculate Ecell for the following reaction. Chemistry questions and answers. Q5 Calculate the emf of the cell in which the following reaction takes place.
Als 3Ag aq Al 3 aq 3Ags. Calculate the value of Ecell at 298K for the following class 12 chemistry CBSE. Calculate E cell for the following reaction at 298 K.
Write the cell reaction and calculate Eo cell of the following electrochemical cell. Ag aq e- Ags 080 V. Al 3 aq 3e- Als -166 V.
6 Calculate E cell for the following reaction. Calculate the standard cell potential Eºcell for the following reaction and indicate if E the reaction is spontaneous or nonspontaneous. Report Ecell in V and in two decimal places.
2FE2 ag Cd2 ag 2FE3 ag Cd s 2. E A g A g 0. 7 Aluminum forms a layer of aluminum oxide when exposed to air which protects the bull 7 metal from further corrosion.
Ni s 2Ag 0002 M Ni2. O 2 g 4 H aq 4 Br-aq 2 H 2 Ol 2 Br 2 l. SAl 1M aqAl3 1M aqZn2 sZn EoAl - 166 V EoZn - 076 V.
Calculate the E cl for the following reactions and state if the given cell is either a galvanic or electrolytic cell. Calculate ECell and ΔrG for the following reaction at 25C. Calculate E o E and ΔG for the following cell reactions a Mg s Sn2 aq Mg2 aq Sn s where Mg2 0025 M and Sn2 0040 M E o V E V ΔG kJ b 3Zn s 2Cr3 aq 3Zn2 aq 2Cr s where Cr3 0040 M and Zn2 00085 M E o V E V ΔG kJ.
Nis Br21 A. Solve Study Textbooks Guides. 8 0 V A g N H 3 2 A g 2 N H 3.
A2 B A3 B if Kc 1010 and 1F 96500 C mol-1. Calculate E cell for the following reaction at 298 K. Calculate the number of moles of electrons transferred in the balanced equation for the cell reaction n for this reaction by determining the half reactions The half reactions are 3Ags rarr 3Agaq 3e-.
Sn4 aq Mn2 aq 2 H20 1 Sn2 aq MnOz s 4H aq Previous question. E 0 cell E 0red cathode - E 0 red anode. Chemistry questions and answers.
Has Eøcell 0236 V at 298 K. Calculate Ecell for the following balanced redox reaction. Q6 The cell in which the following reactions occurs.
- 23138641 caitiebug1701 caitiebug1701 04262021 Chemistry College answered Calculate Ecell for the following balanced redox reaction. Calculate E of the following half-cell reaction at 2 9 8 K. E cell 0261 V.
The cell in which the following reaction occurs. Chemistry questions and answers. A g N H 3 2 e A g 2 N H 3 A g e A g.
4Al s 3028- 2A1203 s Using the thermodynamic data. Use the standard reducti potentials given below. 2Fe3 aq 2I- aq 2Fe2 aq I2 s has E cell 0236 V at 298 KCalculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
2Fe2 aq Cd2 aq 1 2Fe3 aq Cd s A 117 V C-037V D 037 V E None of these. It is given by. Calculate the value of Ecell for the following reaction2Au s 3Ca2 aq 2Au3 aq 3Ca sAu3 aq 3e- Au s E 150VCa2 aq 2e- Ca s E -287VA -437 VB -137 VC -116 VD 137 VE 437 V.
E cell E o cell - 00592n log Q. K 6 1 0 8. Textbook-specific videos for college students.
Calculate the standard cell potential Eºcell for the following reaction. What is the easiest way to calculate E cell values. The overall reaction 2Co 3 aq 2Cl aq.
Solved Calculate Ecell for the following reaction. Calculate the standard cell potential E cell for the following redox reaction. 2Co 2 aq Cl 2 g has the standard cell voltage E cell 046 V.
We review their content and use your feedback to keep the quality high. 2Al s 3Cu 2 001M 2Al 3 001M 3Cu s Given. 2Cr s 3Fe 2 001 M 2Cr 3 001 M 3Fe s Given.
E 0 is the standard EMF of any cell.

Examples Of Chemical Change And How To Recognize It Chemical Changes Chemical Weathering Photosynthesis And Cellular Respiration

Chemistry Notes Chemistry Pdf Electrochemistry And Galvanic Cells Chemistry Notes Electrochemistry Chemistry Lessons

Describing Redox Reactions Handout And Worksheet Redox Reactions Handouts Science Chemistry
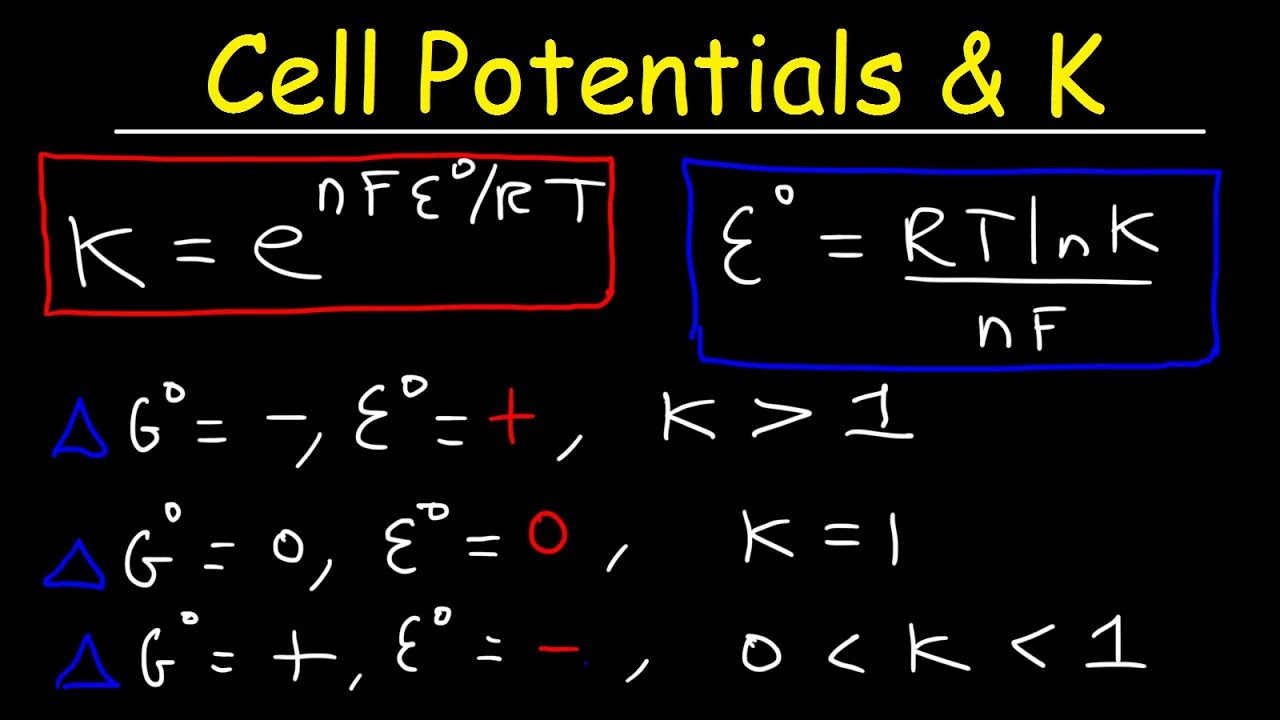
Cell Potential Gibbs Free Energy Standard Reduction Potentials Electrochemistry Problems Youtube

Cell Structure Microscopy Microscopy Lab Activities Cell Structure

Cell Potential Problems Electrochemistry Youtube

Diagramming Galvanic Cells Handout And Worksheet Galvanic Cell Ap Chemistry Electrochemistry

Half Reaction Easy Science Redox Reactions 10th Grade Science Easy Science

Chemistry Notes Chemistry Pdf Electrochemistry And Galvanic Cells Chemistry Notes Electrochemistry Chemistry

Electrochemistry Notes And Galvanic Cell Notes Galvanic Cell Chemistry Notes Electrochemistry

Pin On Gcse Chemistry Revision Notes

Phases Of Reaction Sensitizaiton Phase The Development Of The Dth Response Begins With An Initial Sen Ciencia Del Laboratorio Clinico Immunologia Inmunologia

Emf Of A Cell Definition Formulas Calculation Methods

Nernst Equation And Goldman Hodgkin Katz Voltage Equation Gas Constant Chemistry Lecture Membrane

Galvanic Cells Standard Reduction Potential Ecell Handout And Worksheet Chemistry Worksheets Galvanic Cell Reduction Potential

Calculating E Cell Electromotive Force Emf Youtube

Pin By Engineer Thileban Explains On Chemistry Electrochemistry Chemistry Galvanic Cell

Question Video Calculating The Standard Cell Potential For A Gold Nickel Cell Nagwa
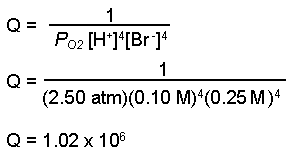
Comments
Post a Comment